|
|
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Primary open angle glaucoma is a chronic progressive disease characterised by damage to the optic nerve head, leading to visual field loss and possible blindness if left untreated.1 Intraocular pressure (IOP) is elevated in the majority of patients, and early intervention, based on IOP reduction, can prevent or delay visual impairment. Topical ocular hypotensive therapy is the mainstay of treatment. Latanoprost is an ester prodrug analogue of prostaglandin F2 Latanoprost Versus An observation-based meta-analysis of 3 randomised double-blind, multicentre phase III trials comparing latanoprost to timolol in patients with open angle glaucoma or ocular hypertension has recently been performed.2 398 patients received latanoprost 0.005% once daily and placebo once daily, while 318 patients received timolol 0.5% twice daily for 6 months. The diurnal IOP was significantly reduced from baseline in both groups: by 8.0 ± 0.1 mm Hg in the latanoprost-treated group (p < 0.001) and by 6.7 ± 0.1 mm Hg in the timolol-treated group (p < 0.001). The difference of 1.3 mm Hg between the 2 groups was statistically significant in favour of latanoprost (p < 0.001). A 30% or greater reduction in intraocular pressure (IOP) was achieved by 61% of patients given latanoprost compared with 40% of those receiving timolol (table 1). Table 1. Percentage of patients achieving reduction in intraocular pressure (IOP) following treatment with latanoprost or timolol for 6 months2
Similarly, a study in the Philippines found that latanoprost 0.005% administered once daily was at least as effective as timolol 0.5% twice daily in reducing the mean diurnal IOP after 6 and 12 weeks of treatment in Filipino patients (table 2).3 Table 2 . Percentage of patients achieving reduction in intraocular pressure (IOP) following treatment with latanoprost or timolol for 6 weeks3
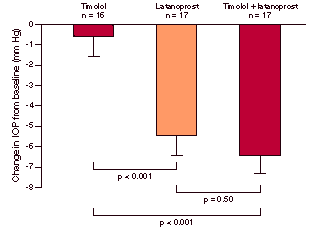
Nordmann et al. found that switching to latanoprost monotherapy in patients not adequately controlled by timolol alone was at least as effective in reducing the mean diurnal IOP as substituting timolol with fixed combination timolol-pilocarpine.4 197 patients, previously treated with ß-adrenergist antagonists alone or in combination, received timolol 0.5% for a run-in period of 21 days, after which the patients were randomised to 2 study groups; 1 group received latanoprost 0.005% once daily while the other received fixed combination timolol 0.5%-pilocarpine 2% twice daily. Switching to latanoprost reduced the diurnal IOP by 5.4 ± 0.3 mm Hg, while timolol-pilocarpine reduced the diurnal IOP by 4.9 ± 0.4 mm Hg. These researchers concluded that switching to latanoprost monotherapy may be attempted before initiating combination therapy. Latanoprost in Several studies have been performed to evaluate the effect of adding latanoprost to treatment for patients with open angle glaucoma or ocular hypertension not adequately controlled by monotherapy alone. 50 patients receiving timolol monotherapy were included in a 6-week double-blind, randomised, multicentre study of timolol alone, latanoprost alone or latanoprost and timolol in combination.5 These researchers found that continuation of timolol monotherapy caused a reduction in IOP of 0.6 mm Hg, while switching to latanoprost mono-therapy or adding latanoprost to timolol both caused significant IOP reductions of 5.0 ± 0.9 mm Hg and 5.9 ± 0.9 mm Hg, respectively (figure 1). Figure 1. Intraocular pressure reduction (mean ± SEM) at 6 weeks compared with baseline. Similarly, Emmerich et al. found that the same diurnal IOP reduction was achieved by switching from timolol to latanoprost as when dorzolamide was added to timolol for patients not adequately controlled by timolol alone.6 Switching from timolol to latanoprost further reduced the diurnal IOP by 4.5 ± 0.2 mm Hg, while adding dorzolamide to timolol reduced the IOP by 4.4 ± 0.2 mm Hg (p < 0.001 for both treatment groups). Bucci et al. found a significantly greater reduction in IOP with latanoprost alone than with pilocarpine and timolol in patients not adequately controlled by timolol alone.7 Long-term Latanoprost The long-term effects of latanoprost have been evaluated in a pooled data analysis.8 In 3 similarly designed phase III studies (from the USA, UK and Scandinavia), patients with open angle glaucoma or ocular hypertension were randomised and treated for 6 months with latanoprost 0.005% once daily or timolol 0.5% twice daily. After completion of the 6-month study period, patients were given the option of subsequent extensions of 6 months and 1 year open-label treatment with latanoprost. A total of 113 patients who were treated with latanoprost monotherapy for 24 months maintained an average IOP reduction of 32% from baseline and the IOP was considered to be sufficiently low in 93% of patients. These authors concluded that the IOP reducing effect of latano-prost may be maintained for at least 24 months. Watson et al. found a similar result in 277 patients treated with latanoprost for up to 24 months.9 Latanoprost significantly reduced IOP by approximately 8 mm Hg from baseline values, and this reduction was maintained throughout the 24-month treatment period (p < 0.001). Cost-effectiveness of Economic evaluation of new treatments in the field of glaucoma is a challenge in the absence of a clear epidemiological link between raised IOP and disease progression.10 However, cost-effectiveness may be expressed as the ability to control IOP over time. A retrospective analysis found that the baseline IOP was highly correlated with costs (the higher the baseline IOP, the more intensive the treatment and the higher the costs), while IOP reduction after treatment initiation was negatively correlated with cost (a better initial treatment effect reduced subsequent costs). Observational data indicate that a high frequency of treatment changes is associated with higher costs. Therefore, new therapies that effectively control IOP over time may reduce the cost of treatment. However, prospective studies are required to confirm this. References
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||